What Is Valence or Valency in Chemistry Valency The combining capacity of an element is known as its valency. Formulae and valencies of common ions Electropositive Ions Monovalent Bivalent Name Formula Name Formula Potassium K+ Barium Ba2+ Sodium Na+ Calcium Ca2+ Copper I Cu+ Magnesium Mg2+ Manganese II Mn2+ Zinc Zn2+ Silver Ag+ Iron II. In chemistry, valence, also known as valency or valence number, is the number of valence bonds 1 a given atom has formed, or can form, with one or more other atoms. For most elements the number of bonds can vary. The IUPAC definition limits valence to the maximum number of univalent atoms that may combine with the atom, that is the maximum number of valence bonds that is possible for the.
Valence Chemistry Related People
In chemistry, valency or valence is the number of chemical bonds that an atom of a certain element can form.
For a long time, people thought that this number was a fixed property of the element in question. They thought that carbon always has four bonds, oxygen always has two, and hydrogen always has one. The problem was seen only later. For example, phosphorous sometimes behaves as if it had three bonds, a valence of three. At other times, though, it seems to have five bonds.
IUPAC saw this problem, and proposed oxidation numbers. This means there is one number per chemical element. The problem with this approach is that it leaves aside most[source?] chemical properties of the elements in question.
A valence band is the highest occupied molecular orbital normally occupied by valence electrons for a given solid at absolute zero temperature.
Related pages[change | change source]
Other websites[change | change source]
Define Valence Chemistry
- 'Valence' from the IUPACGold Book (PDF file)
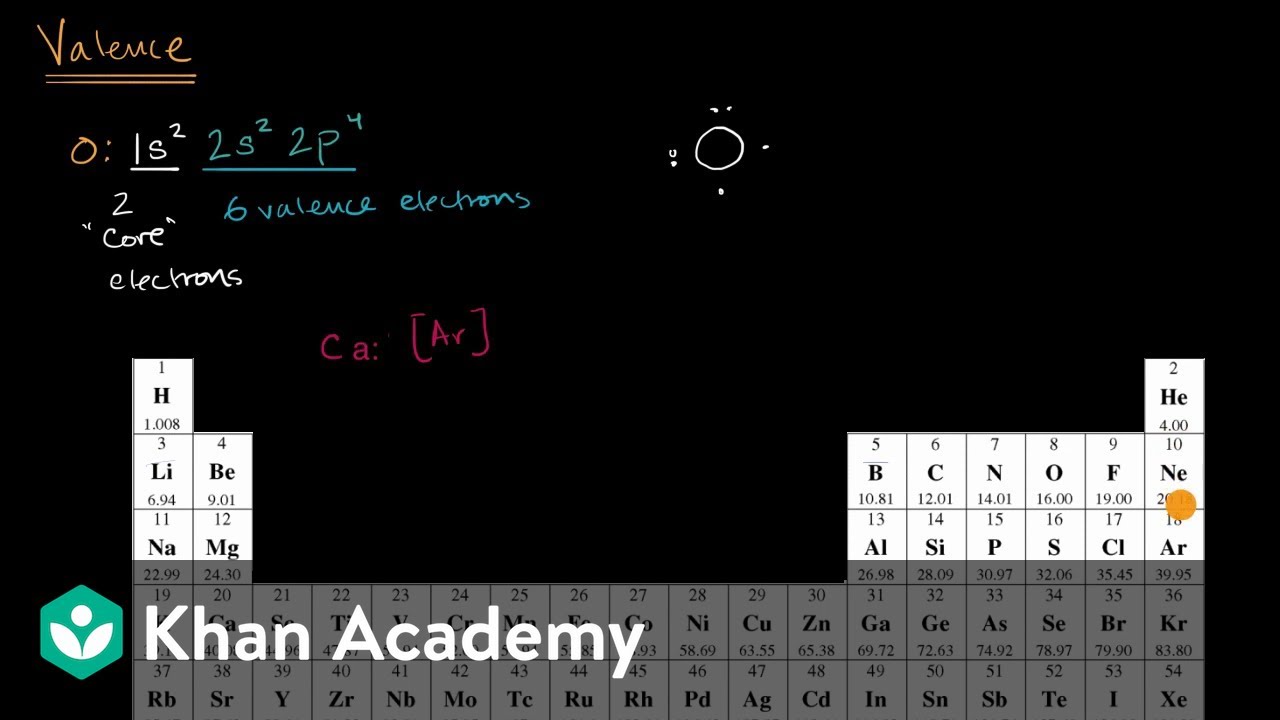
In chemistry, valence, also spelled “valence”, is usually the number of electrons required to fill an atom’s outermost shell. The word, which was introduced in 1868, is used to express both the combined power of an element in general and the numerical value of the combined power. The more general concept of valence is the number of electrons with which a given atom normally binds or the number of bonds that form an atom because there are exceptions. For example, a number of hydrogen compounds such as NH3, N2H4, N3H are formed by nitrogen in which nitrogen atoms have valencies of 3, 2, and 1/3 respectively. This idea of valency as a mere number, therefore, was not clear.
Consequently, valency was later identified as the number of chemical bonds in a molecule formed by an atom. The number of hydrogen atoms that it combines with defines the combining potential or affinity of an atom of a given element. In methane, carbon has a valence of 4; nitrogen has a valence of 3 in ammonia; oxygen has a valence of 2 in water, and chlorine has a valence of 1. in hydrogen chloride. Chlorine can be substituted for hydrogen since it has a valence of one. Valence’s description and systematization was a significant problem for chemists of the 19th century. Most of the effort centered on developing empirical rules for determining the valence of the elements, in the absence of any adequate theory of its cause.
Electronegativity of Valence
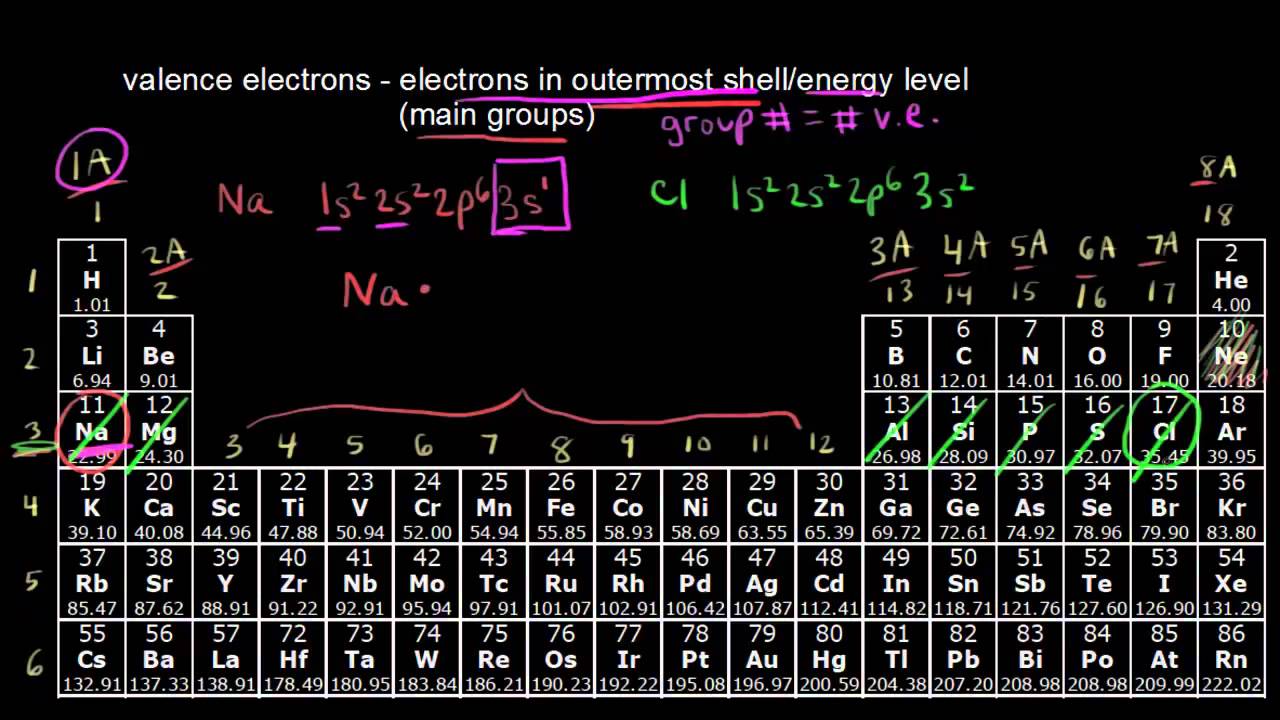
In the main group of the periodic table, atoms of elements can show a valence between 1 and 7 (since 8 is a complete octet).

- Group 1 (I) – Usually displays a valence of 1. Example: Na in NaCl
- Group 2 (II) – Typical valence is 2. Example: Mg in MgCl2
- Group 13 (III) – Usual valence is 3. Example: Al in AlCl3
- Group 14 (IV) – Usual valence is 4. Example: C in CO (double bond) or CH4 (single bonds)
- Group 15 (V) – Usual valences are 3 and 5. Examples are N in NH3 and P in PCl5
- Group 16 (VI) – Typical valences are 2 and 6. Example: O in H2O
- Group 17 (VII) – Usual valences are 1 and 7. Examples: Cl in HCl
In phosphorus pentachloride, PCl5, phosphorus has a 5-valence. The connectivity of the elements is represented by Valence diagrams of a compound, with lines drawn between two elements, also called bonds, indicating a saturated valency for each element. The characteristic valences for the elements were calculated in terms of the number of hydrogen atoms that can be combined or substituted in a compound by an atom of the element. It became obvious, however, that the valences of several elements vary in various compounds. In 1425, from the Latin word valentia, which means strength or power, the word “valence” was defined. To clarify chemical bonding and molecular structure, the principle of valence was developed in the second half of the 19th century.
The American chemist, G.N., took the first significant step in establishing a satisfactory description of valence and chemical combination. Lewis (1916) described the chemical bond of organic compounds with a pair of electrons which hold two atoms together and serve to hold them together. In the same year, the nature of the chemical bond between electrically charged atoms (ions) was discussed by the German physicist W. Kossel. The IUPAC (International Pure and Applied Chemistry Union) describes Valence as:
“The maximum number of univalent atoms (originally hydrogen or chlorine atoms) that may combine with an atom of the element under consideration, or with a fragment, or for which an atom of this element can be substituted.”
In terms of electronic structures and interatomic powers, the theory of valence was reformulated following the development of the detailed electronic theory of the periodic system of the elements. This condition has led to the introduction of many new terms, namely ionic valence, covalence, number of oxidation, number of coordination, metallic valence, corresponding to various modes of atomic interaction. The valence of an element was defined by most chemists of the 19th century as the number of its bonds without distinguishing different forms of valence or bond. However, in 1893 Alfred Werner described transition metal coordination complexes such as (Co(NH3)6)Cl3, in which he distinguished principal and subsidiary valences (German: ‘Hauptvalenz’ and ‘Nebenvalenz’), corresponding to the modern concepts of oxidation state and coordination number respectively.
Valency is different from the number of oxidation, and it has NO Symbol. Hence, nitrogen valency is 3, while oxidation numbers can range from -3 to +5. The oxidation number is the hypothetical charge in a molecule or ion of an atom, and it is a measure of its apparent capacity within that species to gain or lose electrons. Many elements have a particular valence associated with their place in the periodic table, and the octet rule rationalizes this nowadays. A positive charge can be gained by donating an electron and a negative charge vice versa by loss of electron or electron gain called atom charge. So Valence has no symbol, there are both positive and negative signs for Fee.
The importance of valence: The outer shell first comes into contact when two atoms react and it is, thus, the outer shell electron that is usually involved in a chemical reaction. In order to balance their valence shell, the atoms exchange valence electrons in a chemical reaction. Other notations are currently favored owing to the uncertainty of the word valence. In addition to the scheme of oxidation numbers used in the Coordination Compound Stock Nomenclature and the lambda notation used in the Inorganic Chemistry IUPAC Nomenclature, the oxidation state is a better representation of the electronic state of atoms in the molecule. The valence of elements in a high oxidation state can be greater than four. For instance, chlorine has seven valence bonds in perchlorates; ruthenium has eight valence bonds in the +8 oxidation state in ruthenium tetroxide.
Chemical Valence
Information Sources:
